Have you ever wondered how cells transport molecules across their membranes? How do substances move in and out of cells, ensuring the proper functioning of living organisms? The answer lies in the fascinating processes of diffusion and osmosis. These fundamental mechanisms play a crucial role in maintaining cellular homeostasis and facilitating various biological processes. In this article, we will delve into the virtual lab answer key for diffusion and osmosis, unlocking the secrets behind these essential cellular activities.
Understanding Diffusion
The Basics
Diffusion, like the aroma of your favorite pizza wafting through the air, is the spontaneous movement of particles from an area of high concentration to an area of low concentration. It is a passive process that does not require any energy expenditure from the cell. This natural tendency for molecules to spread out and mix evenly is driven by the concept of entropy, or the measure of disorder in a system.
In the context of cellular transport, diffusion allows various substances, such as oxygen and carbon dioxide, to move freely across the cell membrane. Through simple diffusion, small nonpolar molecules can easily pass through the lipid bilayer, while larger or charged molecules require specialized transport proteins.
The Virtual Lab Answer Key
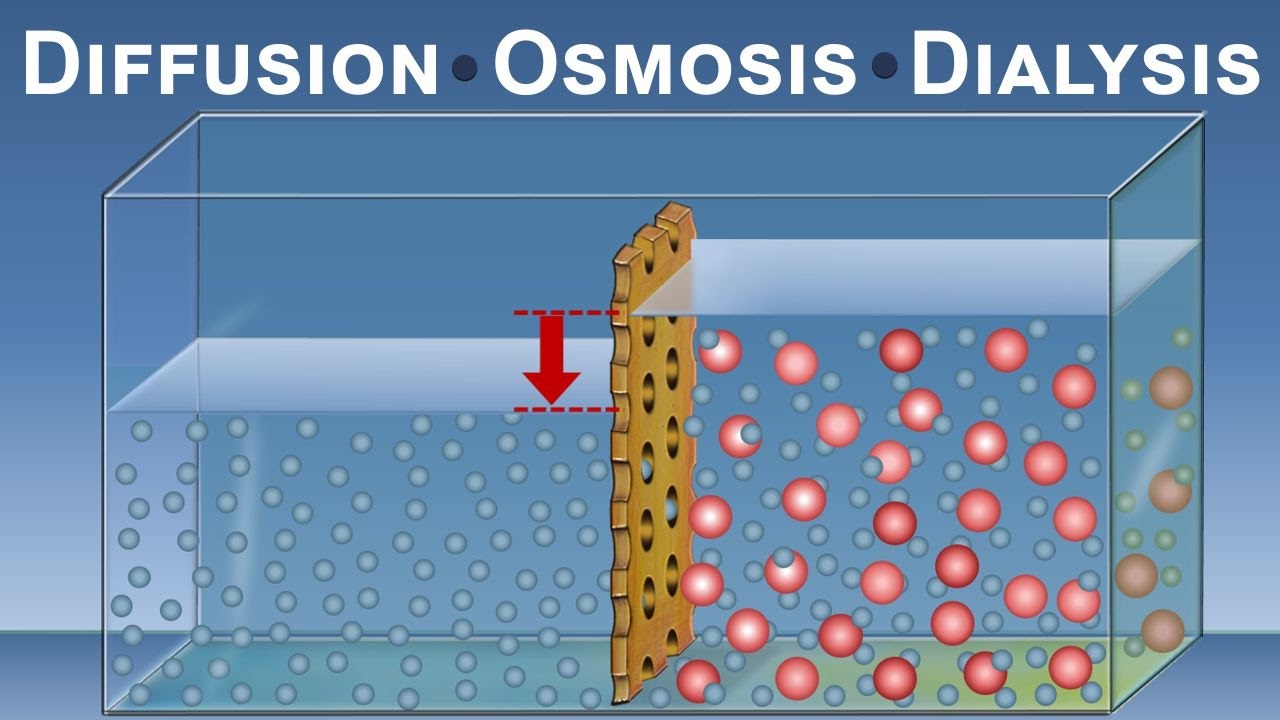
In the diffusion virtual lab, you were presented with a scenario where a solute was dissolved in a solvent. The solute particles were initially confined to one side of a semi-permeable membrane, while the solvent molecules were present on both sides. As time progressed, the solute particles began to move, spreading out and eventually reaching an equilibrium.
The key to understanding the results lies in the concept of concentration gradients. At the beginning of the experiment, there was a high concentration of solute particles on one side, creating a concentration gradient across the membrane. As the solute particles diffused, they gradually became evenly distributed on both sides, resulting in a state of equilibrium.
The diffusion virtual lab answer key reveals that the rate of diffusion is influenced by several factors, including the size of the particles, temperature, and the concentration gradient. Smaller particles diffuse more rapidly, as they have a higher probability of colliding with the membrane. Similarly, an increase in temperature enhances the kinetic energy of the particles, accelerating their movement. Lastly, a steeper concentration gradient leads to faster diffusion, as there is a greater difference in solute concentrations.
Unveiling the Mysteries of Osmosis
The Phenomenon
While diffusion deals with the movement of solute particles, osmosis focuses on the movement of water molecules across a semi-permeable membrane. Osmosis occurs when there is a difference in solute concentrations on either side of the membrane, resulting in the net movement of water from an area of lower solute concentration to an area of higher solute concentration.
Think of osmosis as a thirsty traveler stumbling upon an oasis in the desert. The traveler, representing water molecules, will naturally flow towards the higher concentration of solutes, seeking equilibrium. This process is essential for maintaining the proper balance of water within cells and ensuring their survival.
Navigating the Osmosis Virtual Lab Answer Key
In the osmosis virtual lab, you were tasked with observing the movement of water molecules across a selectively permeable membrane. The key findings from the virtual lab answer key shed light on the influence of solute concentration and membrane permeability on osmosis.
When the solute concentration outside the cell is higher than inside, the solution is said to be hypertonic. In this scenario, water molecules will move out of the cell, causing it to shrink. On the other hand, when the solute concentration inside the cell is higher, the solution is hypotonic. Water molecules will rush into the cell, causing it to swell and potentially burst.
The virtual lab answer key also demonstrates the concept of isotonic solutions, where the solute concentration is the same inside and outside the cell. In this case, there is no net movement of water, and the cell remains at equilibrium.
Frequently Asked Questions
Q: How do diffusion and osmosis differ?
A: Diffusion involves the movement of solute particles from an area of high concentration to low concentration, while osmosis specifically refers to the movement of water molecules across a semi-permeable membrane.
Q: Are diffusion and osmosis active processes?
A: Diffusion is a passive process, requiring no energy expenditure, while osmosis is also a passive process but involves the movement of water molecules.
Q: Can you provide examples of diffusion and osmosis in everyday life?
A: Diffusion can be observed when the smell of freshly baked cookies spreads throughout a room. Osmosis is evident when you soak a raisin in water, causing it to plump up as water molecules move into the raisin.
Q: How do cells regulate osmosis?
A: Cells maintain osmotic balance through specialized transport proteins and ion channels, controlling the movement of water and solute molecules.
Conclusion
In conclusion, the diffusion and osmosis virtual lab answer key has provided us with valuable insights into the mysteries of cellular transport. Diffusion allows molecules to move from areas of high concentration to areas of low concentration, driven by the natural tendency for particles to spread out. Osmosis, on the other hand, focuses on the movement of water across a semi-permeable membrane, ensuring the proper balance of solutes and water within cells.
Through the virtual lab, we have explored the factors influencing diffusion, such as particle size, temperature, and concentration gradient. We have also witnessed the impact of solute concentration and membrane permeability on osmosis. These findings deepen our understanding of cellular processes and highlight the vital role played by diffusion and osmosis in maintaining cellular homeostasis.
So, the next time you enjoy the aroma of your favorite pizza or witness the plumpness of a soaked raisin, remember that diffusion and osmosis are at work, orchestrating the intricate dance of cellular transport.