Have you ever wondered about the fascinating world of physics and its intricate concepts? One such concept that continues to captivate scientists and researchers alike is the photoelectric effect. This phenomenon, first discovered by Albert Einstein, has revolutionized our understanding of light and electrons. In this article, we will delve into the realm of the photoelectric effect virtual lab, uncovering its significance and providing answers to common questions that arise during the exploration of this captivating subject.
Understanding the Photoelectric Effect
What is the Photoelectric Effect?
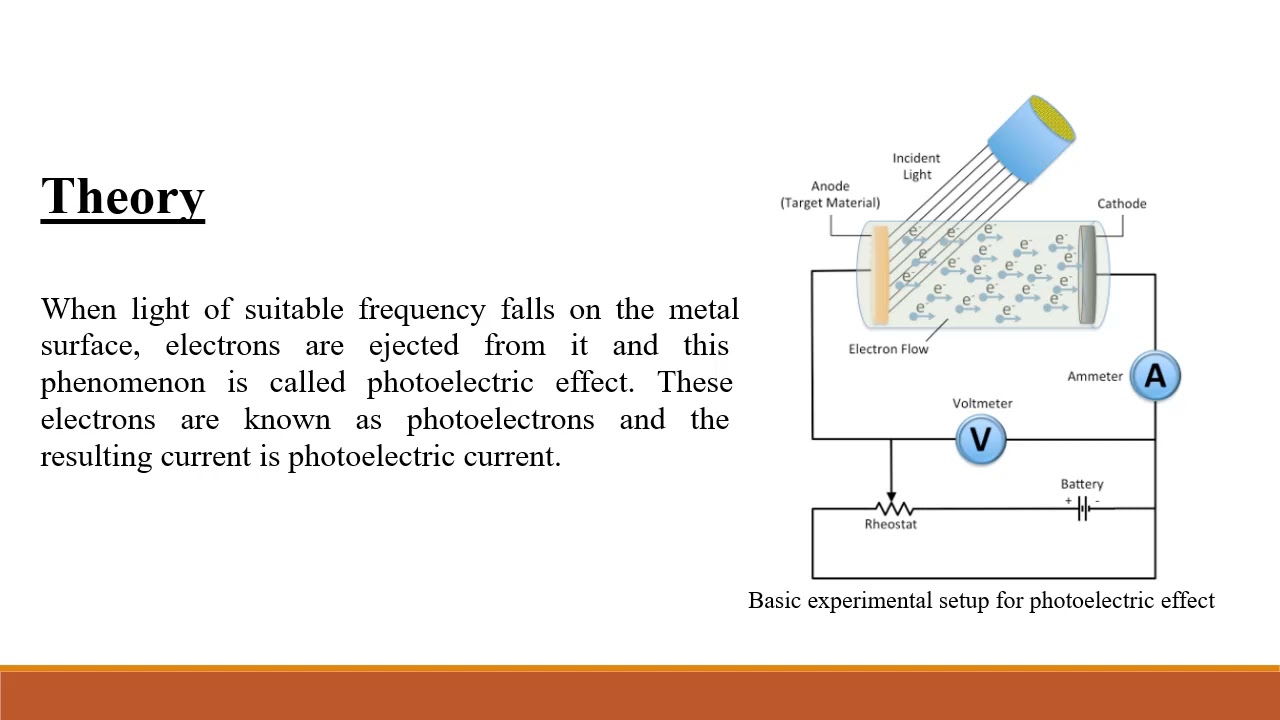
The photoelectric effect refers to the emission of electrons from a material’s surface when it is illuminated by light. This groundbreaking discovery, made by Heinrich Hertz in 1887 and later explained by Albert Einstein in 1905, challenged the traditional wave theory of light and paved the way for the quantum theory. According to Einstein’s explanation, light behaves as discrete particles called photons, each carrying a specific amount of energy. When these photons strike a material’s surface, they may transfer enough energy to electrons, enabling them to overcome the binding forces and escape the material.
Importance of the Photoelectric Effect
The photoelectric effect holds immense significance in various fields, ranging from physics and chemistry to engineering and technology. Understanding this phenomenon has paved the way for numerous scientific advancements, from the development of solar cells and photodiodes to the invention of photoelectric sensors and night-vision devices. The photoelectric effect also plays a crucial role in understanding the behavior of electromagnetic radiation, contributing to the development of quantum mechanics.
Exploring the Photoelectric Effect Virtual Lab
What is a Virtual Lab?
A virtual lab, as the name suggests, is a simulated environment that allows users to perform experiments and explore scientific concepts without the need for physical equipment or resources. In the context of the photoelectric effect, a virtual lab provides a virtual platform where users can observe and analyze the effect, conduct experiments, and draw conclusions just as they would in a traditional laboratory setting.
Benefits of a Photoelectric Effect Virtual Lab
A virtual lab offers several advantages over traditional labs. Firstly, it provides a safe and controlled environment for experimentation, eliminating potential hazards and risks associated with handling physical equipment. Additionally, a virtual lab allows for easy repetition of experiments, enabling users to validate their findings and gain a deeper understanding of the photoelectric effect. Moreover, virtual labs are accessible from anywhere, anytime, making them an invaluable resource for both students and researchers.
Conducting Experiments in the Virtual Lab
When conducting experiments in the photoelectric effect virtual lab, users can manipulate various parameters, such as the intensity and wavelength of light, as well as the type of material being used. By altering these parameters, users can observe changes in the emission of electrons and analyze the relationship between the incident light and the resulting photoelectric effect.
Virtual Lab Answers to Frequently Asked Questions
Q1: How does the intensity of light affect the photoelectric effect?
A1: In the virtual lab, you may have noticed that increasing the intensity of light leads to a higher number of emitted electrons. This is because the intensity of light directly corresponds to the number of photons striking the material’s surface. As the number of photons increases, more electrons receive the necessary energy to overcome the binding forces and escape the material. Therefore, higher intensity light results in a more pronounced photoelectric effect.
Q2: What is the role of wavelength in the photoelectric effect?
A2: The wavelength of light influences the energy carried by each photon. In the virtual lab, you may have observed that changing the wavelength of light affects the kinetic energy of emitted electrons. Shorter wavelengths, such as ultraviolet light, carry higher energy photons compared to longer wavelengths, such as infrared light. Therefore, changing the wavelength enables users to explore the relationship between photon energy and the photoelectric effect.
Q3: Can you explain the concept of the work function?
A3: Certainly! The work function, denoted by the symbol φ (phi), represents the minimum amount of energy required to remove an electron from a material’s surface. It depends on the material’s properties and can be thought of as the energy barrier that electrons must overcome to escape the material. In the virtual lab, you can explore how different materials have different work functions, leading to variations in the ease with which photoemission occurs.
Conclusion
In conclusion, the photoelectric effect virtual lab offers an immersive and interactive experience for exploring the captivating phenomenon of the photoelectric effect. Through manipulating various parameters such as light intensity, wavelength, and material type, users can observe and analyze the emission of electrons and gain a deeper understanding of this fundamental concept. The virtual lab serves as a valuable tool in both education and research, providing a safe, accessible, and repeatable environment for conducting experiments. So, why wait? Dive into the photoelectric effect virtual lab and unravel the mysteries of light and electrons at your fingertips!