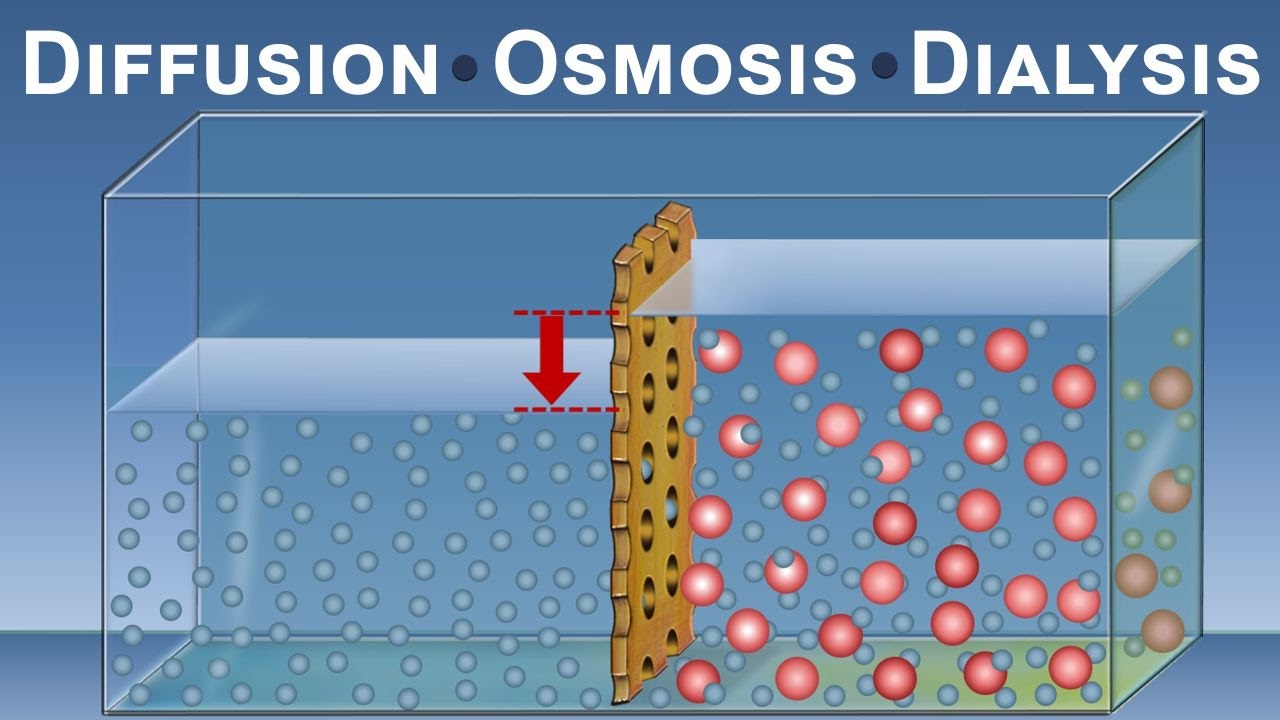
Welcome, avid learners, to our virtual laboratory where we will dive into the captivating world of diffusion. In this article, we will explore the diffusion virtual lab answer key, unlocking the secrets behind the movement of molecules and the factors influencing this process. So, grab your lab coats, put on your safety goggles, and let’s embark on this scientific adventure!
The Basics of Diffusion
Before we delve into the answer key, let’s ensure we have a solid foundation of knowledge about diffusion. Picture this: you’re sitting in a room, and suddenly a whiff of aromatic coffee reaches your nose. How did that scent manage to travel from the coffee pot to your nostrils without any visible assistance? The answer lies in the remarkable phenomenon of diffusion.
Diffusion is the spontaneous movement of molecules from an area of higher concentration to an area of lower concentration. It’s like a wild dance party where molecules jive their way from a crowded space to an empty one. This process occurs due to the inherent random motion of particles, seeking equilibrium and a state of balance.
The Diffusion Virtual Lab Experience
Now that we have a grasp of the basics, let’s uncover the secrets of the diffusion virtual lab. In this immersive experience, you will be transported to a virtual laboratory equipped with cutting-edge technology. Through interactive simulations, you will conduct experiments, make observations, and analyze data to unlock the mysteries of diffusion.
Conducting the Experiments
In the first experiment, you will explore the effect of temperature on the rate of diffusion. As you heat up the system, the molecules gain energy, resulting in increased motion and faster diffusion. Conversely, when the temperature drops, the molecules slow down, leading to a decrease in diffusion rate. So, remember to add some heat to the party if you want those molecules to boogie faster!
Next, you will investigate the impact of molecular size on diffusion rate. Imagine a crowded dance floor where smaller dancers can slide through the gaps more easily than larger ones. Similarly, smaller molecules diffuse faster due to their ability to maneuver through spaces between larger molecules. It’s all about finding the perfect groove!
Analyzing the Data
Now that you’ve completed the experiments, it’s time to analyze the data and unlock the answers hidden within. In the diffusion virtual lab answer key, you will find a comprehensive breakdown of the results, allowing you to draw meaningful conclusions and deepen your understanding of diffusion.
FAQs (Frequently Asked Questions)
Q: Can diffusion occur in any state of matter?
A: Absolutely! Diffusion is not picky when it comes to states of matter. It can occur in gases, liquids, and even solids, albeit at a slower rate.
Q: How does concentration affect diffusion?
A: Concentration plays a vital role in diffusion. The greater the difference in concentration between two areas, the faster the rate of diffusion. It’s like a crowd rushing toward the stage when they hear their favorite band is about to perform!
Conclusion
In conclusion, the diffusion virtual lab answer key has provided us with a wealth of knowledge about the fascinating world of molecular movement. Through the experiments and data analysis, we have unraveled the mysteries of diffusion, understanding factors such as temperature and molecular size that influence the rate of diffusion.
Remember, science is all about curiosity and exploration. So, whether you’re in a virtual lab or a physical one, keep asking questions, conducting experiments, and expanding your understanding of the world around you. Happy diffusing!
Note: The diffusion virtual lab answer key is a crucial resource that guides us through the virtual lab and helps us unlock the secrets of diffusion. Utilize it wisely to enhance your learning experience.