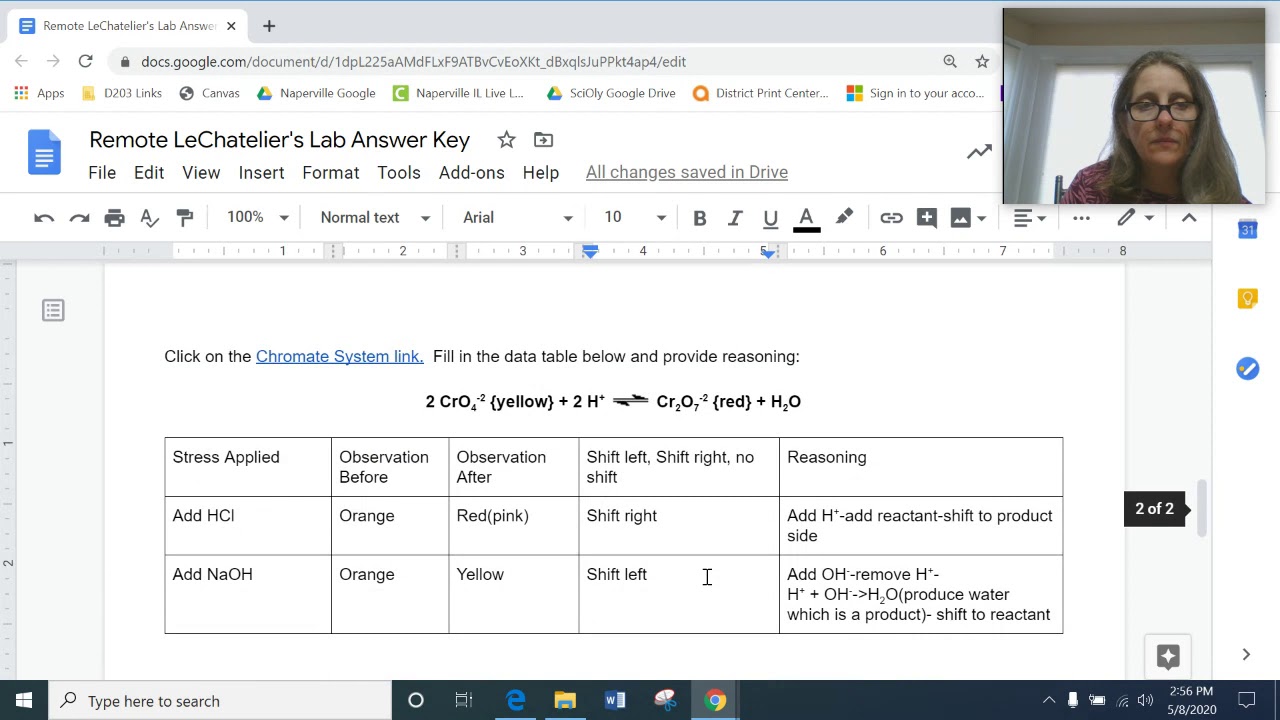
Welcome to our virtual lab where we will dive into the fascinating world of Le Chatelier’s Principle and its application in chemical equilibrium. In this article, we will explore the concept of Le Chatelier’s Principle, discuss its significance in understanding chemical reactions, and provide answers to common questions that arise during virtual lab experiments.
Understanding Le Chatelier’s Principle
Le Chatelier’s Principle is a fundamental concept in chemistry that helps us predict how a system at equilibrium will respond to changes in temperature, pressure, or concentration. It states that when a system in equilibrium is subjected to an external stress, it will adjust itself to counteract the effect of that stress and restore equilibrium.
Before we delve deeper into Le Chatelier’s Principle, let’s first understand what chemical equilibrium is. Chemical equilibrium occurs when the rates of the forward and reverse reactions in a chemical system are equal, resulting in a stable state where the concentrations of reactants and products remain constant over time.
In a virtual lab setting, we can simulate various scenarios to observe and analyze how a system at equilibrium responds to changes. Let’s explore some common questions that arise during virtual lab experiments and provide answers based on Le Chatelier’s Principle.
FAQ
Q1: What happens to the equilibrium if we increase the concentration of a reactant?
When the concentration of a reactant is increased, Le Chatelier’s Principle tells us that the system will shift in the direction that reduces the concentration of that reactant. This means that more products will be formed to restore equilibrium. Conversely, if the concentration of a reactant is decreased, the system will shift to favor the side with more reactants.
Q2: How does temperature affect the equilibrium?
Temperature plays a crucial role in determining the direction of the equilibrium shift. According to Le Chatelier’s Principle, if the forward reaction is exothermic (releases heat), increasing the temperature will favor the reverse reaction to absorb the excess heat. On the other hand, if the forward reaction is endothermic (absorbs heat), increasing the temperature will favor the forward reaction to absorb more heat.
Q3: What happens when we change the pressure of a system?
For systems involving gases, changes in pressure can influence the equilibrium position. If the pressure is increased, the system will shift to the side with fewer moles of gas to reduce the pressure. Conversely, if the pressure is decreased, the system will shift to the side with more moles of gas to restore equilibrium.
Q4: Can catalysts affect the equilibrium?
No, catalysts do not affect the equilibrium position. They increase the rate of both the forward and reverse reactions equally, allowing the system to reach equilibrium faster. However, they do not shift the equilibrium in any particular direction.
Conclusion
In conclusion, Le Chatelier’s Principle is a powerful tool that helps us understand the behavior of chemical equilibrium in response to external changes. Through virtual lab experiments, we can simulate and observe these changes, gaining valuable insights into the principles governing chemical reactions.
By understanding how changes in concentration, temperature, and pressure influence the equilibrium position, we can make predictions about the direction of the equilibrium shift and the resulting changes in the concentrations of reactants and products.
So, next time you enter a virtual lab and encounter questions about Le Chatelier’s Principle, remember that it is all about seeking balance in the face of external stress. Now, armed with this knowledge, go forth and explore the wonders of chemical equilibrium in the virtual realm!
Remember, Le Chatelier’s Principle virtual lab answers can guide you through the intricacies of chemical equilibrium and help you unlock a deeper understanding of this fascinating subject. Happy experimenting!
Note: The content of this article is for educational purposes only and should not replace actual laboratory experiments or professional guidance.